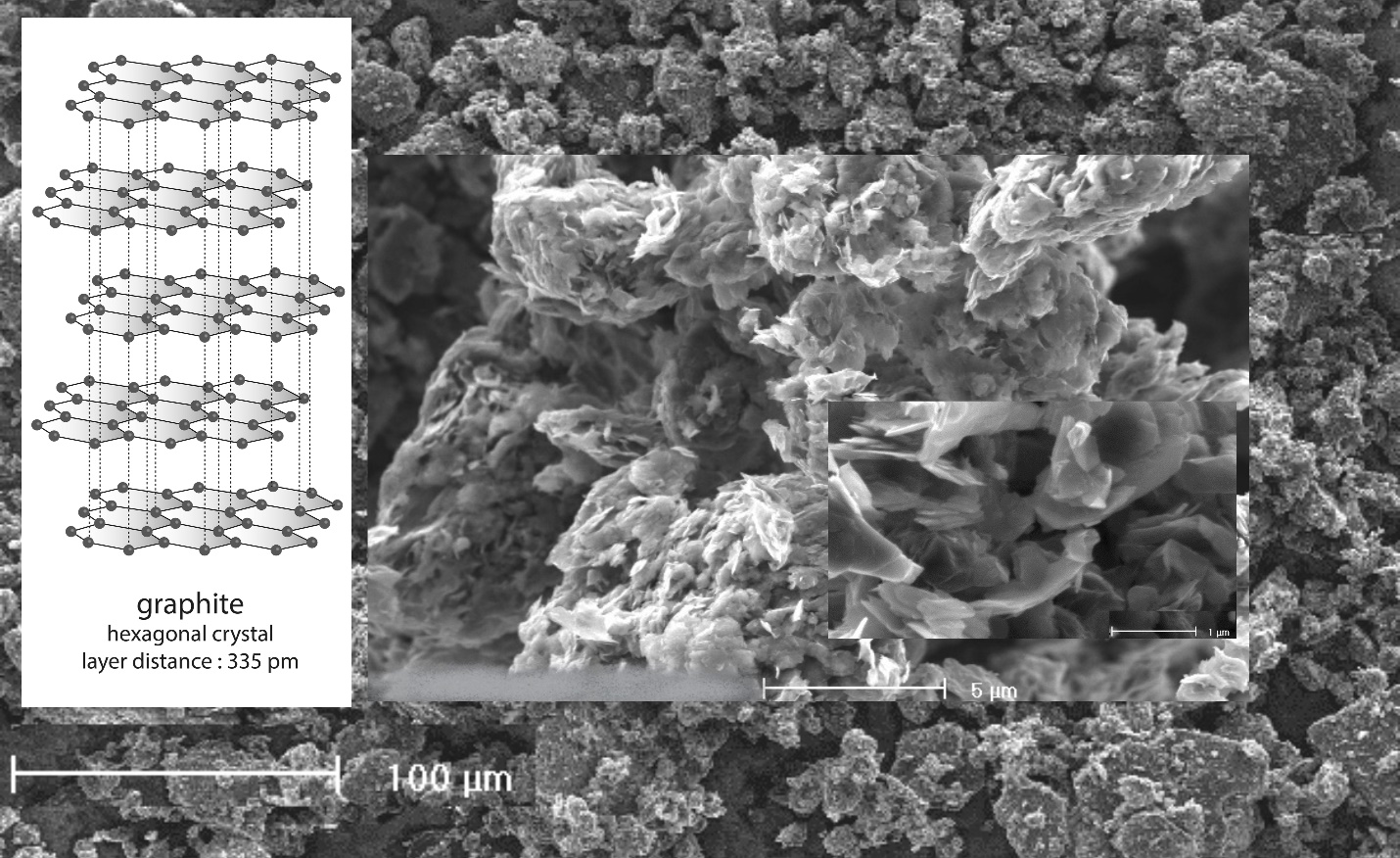
Graphite is one of two natural forms of carbon, the second one is diamond.
It is an only excellent conductor of heat and electricity if pure (without structural defect) & with the lateral plane and a non-metallic mineral.
Graphite is highly refractory, remains stable through a wide range of temperatures (melting point: 3650°C) and holds the highest natural strength and stiffness of any material.
Graphite is non-corrosive and an excellent lubricant due to its lamellar structure. It is an important component in the production of the anode in a Lithium-ion (Li-ion) batteries.
There are many known graphite applications and many more are yet to discover.
Graphite (several layers of graphene) is an amazing material with applications like lubricants, Li-ion batteries, composites, specialty coatings, Graphene manufacturing etc.
The combination of mechanical, thermal and electrical properties makes Graphite an amazing material used in many known applications and also many more applications about to discover.
There are several type and grades of Graphite available in a market based on their source and then carbon content, particle size etc.
• Natural Flake Graphite also known as crystalline graphite
• Amorphous natural graphite also known as microcrystalline graphite
• Synthetic graphite
